
The below infographic summarizes the differences between beryllium and lithium.īoth beryllium and lithium are in the same period, period 2. Beryllium forms divalent cations wile Lithium forms monovalent cations. The key difference between beryllium and lithium is that beryllium is a white-grey metal which is diamagnetic, whereas lithium is a silvery-grey metal which is paramagnetic. Therefore, they have different properties. But, they are in two different groups of the periodic table. What is the Difference Between Beryllium and Lithium?īoth beryllium and lithium are in the same period, period 2. Besides, it has the least density among solid metals. It is the smallest element among other members of this group. For example, it is the only alkali metal that can react with nitrogen gas, and it forms lithium nitride upon this reaction. Moreover, this metal has some unique properties other alkali metals do not have. Also, it can float on water, and thus, resulting in an explosive chemical reaction. Hence, we can cut it easily using a knife. It appears in silvery-white colour, and if we burn this metal, it gives a crimson coloured flame.įurthermore, this metal is very light and soft. Moreover, it belongs to the s block since it is in group 1 of the periodic table and the melting and boiling points of this element are 180.50 ☌ and 1330 ☌, respectively.

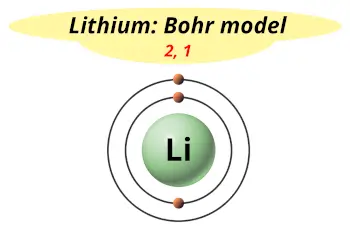
The atomic weight of this element is 6.941, and the electron configuration is 2s 1. According to the big bang theory of the creation of the earth, lithium, hydrogen and helium are the major chemical elements produced at the earliest stages of the world creation. Lithium is an alkali metal having atomic number 3 and chemical symbol Li. There are two main ores of beryllium: beryl and bertrandite What is Lithium? It is present in water, but in very trace amounts. We can mostly observe this metal is sand. When considering the occurrence of beryllium, it has been found that the sun has about 0.1 ppb of beryllium while the concentration of beryllium on earth is about 2-4 ppm. This metal is diamagnetic because it has no unpaired electrons in its ground state. Moreover, the elasticity of beryllium is greater than that of steel.

The melting point of this metal is very high. The stiffness of beryllium is said to be exceptional. It has a close-packed hexagonal crystal system. According to its electron configuration, beryllium forms divalent cations by removing two electrons from the 2s orbital.īeryllium is a hard and brittle metal.
The electron configuration of this element is 2s 2. It is an s-block element because it has its valence electrons in the s orbital. Relatively, it is a rare chemical element in the universe. Side by Side Comparison – Beryllium vs Lithium in Tabular Formīeryllium is an alkaline earth metal having the atomic number 4 and chemical symbol Be. Both these chemical elements are s-block elements because they have their valence electrons in s orbitals. However, they are in two different groups in the periodic table. The key difference between beryllium and lithium is that beryllium is a white-grey metal that is diamagnetic, whereas lithium is a silvery-grey metal that is paramagnetic.īoth beryllium and lithium are in the same period, period 2.


 0 kommentar(er)
0 kommentar(er)
